Manufacturing News of Note—Novartis to have Cell Therapies make Kymriah in Australia; Piramal Pharma expands API plant in Canada | Fierce Pharma
A New Approach to Treat Childhood Leukemia: Novartis' CAR-T Therapy - Frazer A. Tessema, Jonathan J. Darrow, 2017
A New Approach to Treat Childhood Leukemia: Novartis' CAR-T Therapy | Journal of Law, Medicine & Ethics | Cambridge Core

Current state of U.S. Food and Drug Administration regulation for cellular and gene therapy products: potential cures on the horizon - Cytotherapy

NICE backs Novartis' Adakveo via special channel despite 'high uncertainty' about cost, long-term efficacy | Fierce Pharma

ASCO: Gilead's Kite soars over Novartis' CAR-T turf with Tecartus win in type of leukemia | Fierce Pharma

A New Approach to Treat Childhood Leukemia: Novartis' CAR-T Therapy - Frazer A. Tessema, Jonathan J. Darrow, 2017
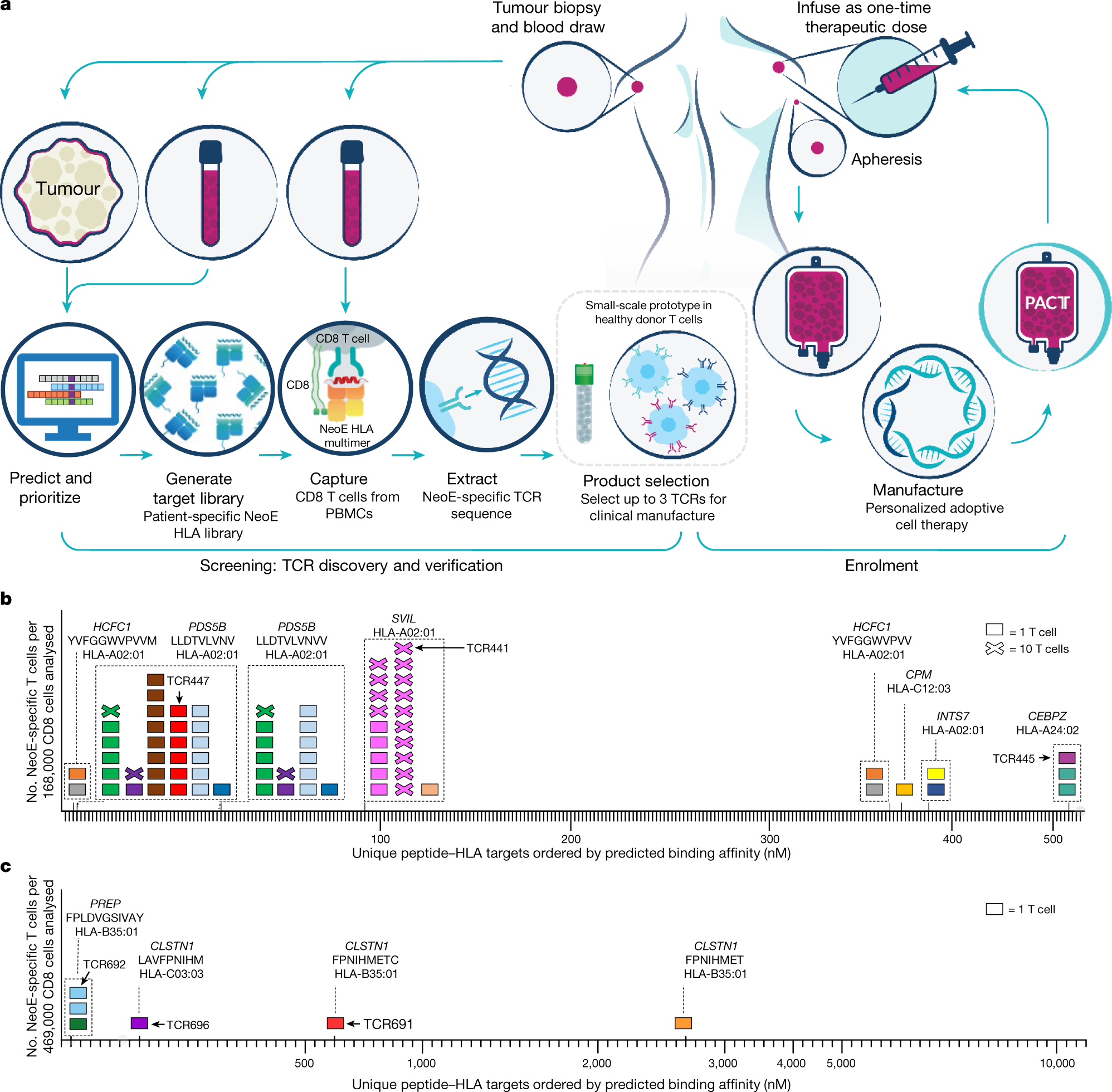
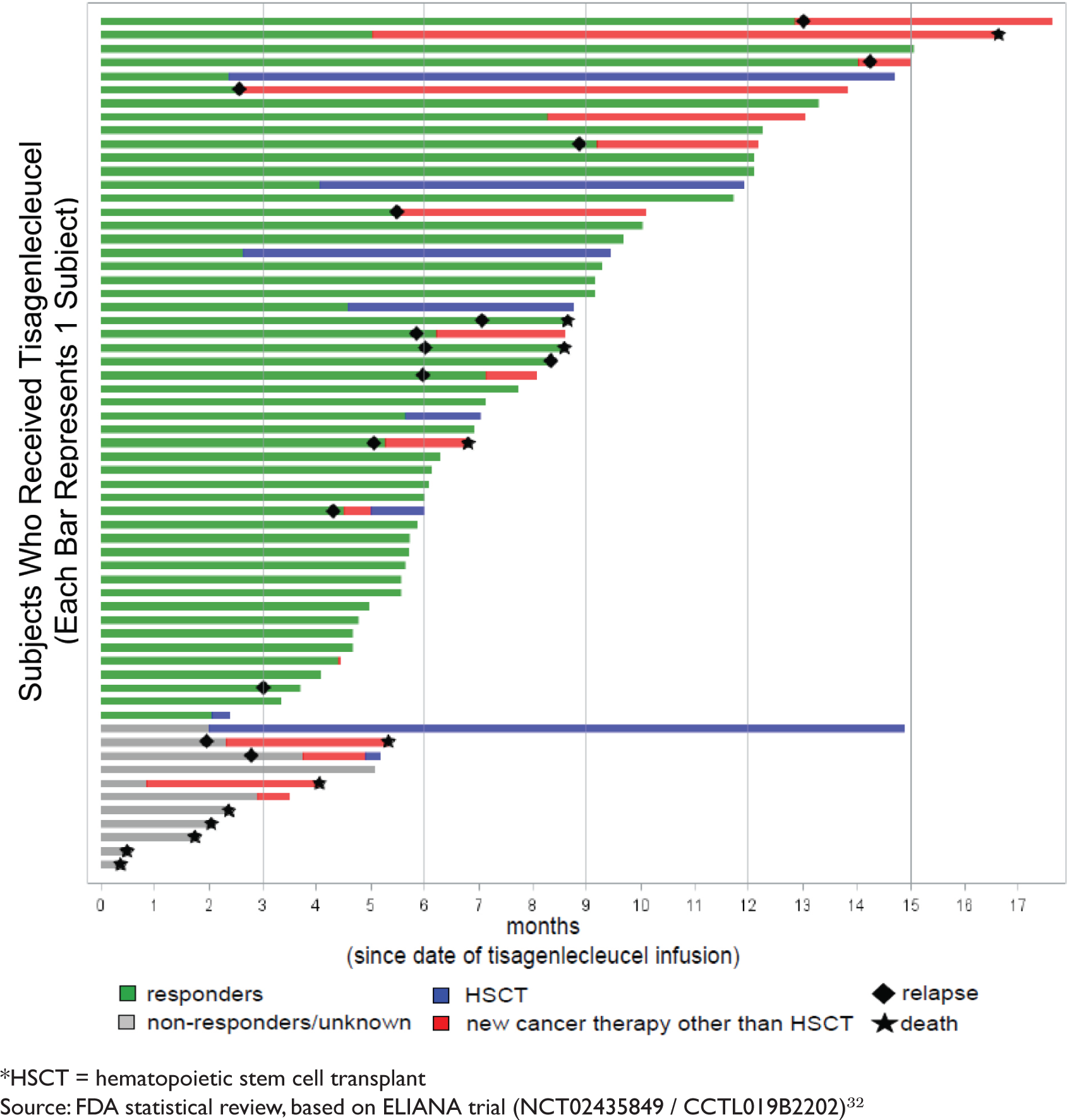
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar
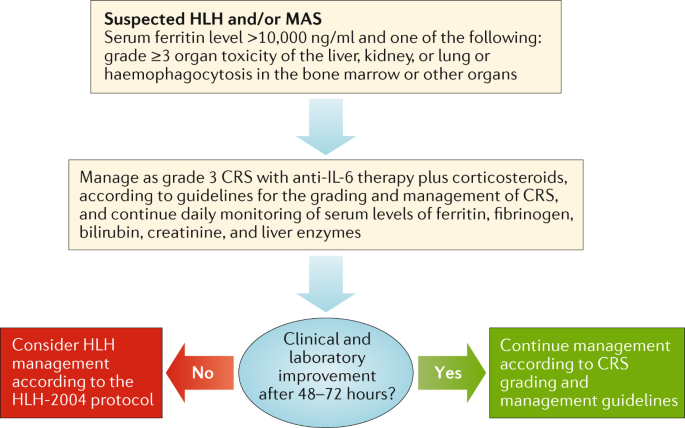
Management guidelines for paediatric patients receiving chimeric antigen receptor T cell therapy | Nature Reviews Clinical Oncology

Characteristics of anti-CD19 CAR T cell infusion products associated with efficacy and toxicity in patients with large B cell lymphomas | Nature Medicine
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar
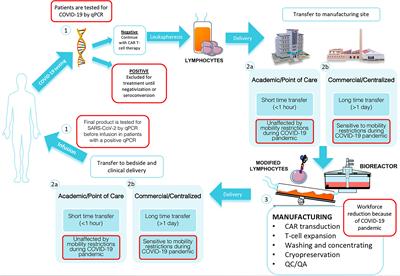
Frontiers | Manufacturing and Management of CAR T-Cell Therapy in “COVID-19's Time”: Central Versus Point of Care Proposals
ONCOLOGIC DRUGS ADVISORY COMMITTEE BRIEFING DOCUMENT Tisagenlecleucel (CTL019) for the TREATMENT OF PEDIATRIC AND YOUNG ADULT PA
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar

Optimizing CAR-T Cell Manufacturing Processes during Pivotal Clinical Trials: Molecular Therapy - Methods & Clinical Development
ASCO: Positive trial shows Novartis' Kymriah poised to play catch-up in CAR- T rivalry with Gilead's Yescarta | Fierce Pharma